Cast iron is a complex multi-component alloy based on carbon, iron, and silicon. Its carbon content (mass fraction) is generally in the range of 2.0%-4.0%. In addition to carbon and silicon, there are also elements such as manganese, phosphorus, and sulfur in cast iron. In order to meet the needs of its work, we often use medium frequency induction heating equipment for heat treatment, and the effect is very good. Today, we won’t talk about its heat treatment process. Let’s look at the changes in its organization when heated.
The as-cast structure of cast iron mainly includes three types: ferrite + graphite, ferrite + pearlite + graphite, and pearlite + graphite. The transformation of the cast iron structure during heating can be summarized into three aspects.
(1) When heating below the critical temperature (lower limit of Ac1), eutectoid cementite begins to spheroidize and graphitize. The slower the heating speed, the more intense the spheroidization and graphitization proceed: as the heating temperature increases, the eutectoid cementite begins to spheroidize and graphitize. The decomposition rate increases and the amount of pearlite decreases. Silicon in cast iron is a graphitizing element that can promote the graphitization process, while manganese, phosphorus, chromium, etc. are elements that stabilize carbides and inhibit the graphitization process and are conducive to the granulation of pearlite.
(2) When heating within the critical temperature range, when the heating temperature exceeds the lower limit of the critical temperature Ac1, the phase transformation process of ferrite to austenite begins. Within the critical temperature range, ferrite, austenite, Three phases of graphite (stable system) or cementite (quasi-stable system) coexist. As the heating temperature increases, the amount of austenite gradually increases, while the amount of ferrite decreases accordingly, until the upper limit temperature of Ac1 is heated, and the ferrite disappears completely. The formation process of austenite in cast iron conforms to the general phase transformation laws, that is, nucleation and growth.
(3) When the heating temperature exceeds the upper limit of the critical temperature Ac1, the ferrite and pearlite are completely austenitized, and the free (primary) cementite present in the original structure of the cast iron decomposes into austenite and graphite, that is, high-temperature graphitization . As the heating temperature increases, the graphitization process accelerates, and part of the carbon on the surface of the graphite will dissolve into the austenite, causing the carbon content in the austenite to increase. At the same time, increasing temperature causes austenite grain growth and graphite aggregation. The alloying elements carbon, silicon, copper, aluminum, nickel, etc. in cast iron promote the graphitization process and accelerate the decomposition of cementite. Elements that stabilize carbides such as chromium, molybdenum, vanadium, and sulfur reduce the decomposition rate of cementite.




 en
en  cn
cn  jp
jp  ko
ko  de
de  es
es  it
it  ru
ru  pt
pt  vi
vi  th
th  pl
pl 




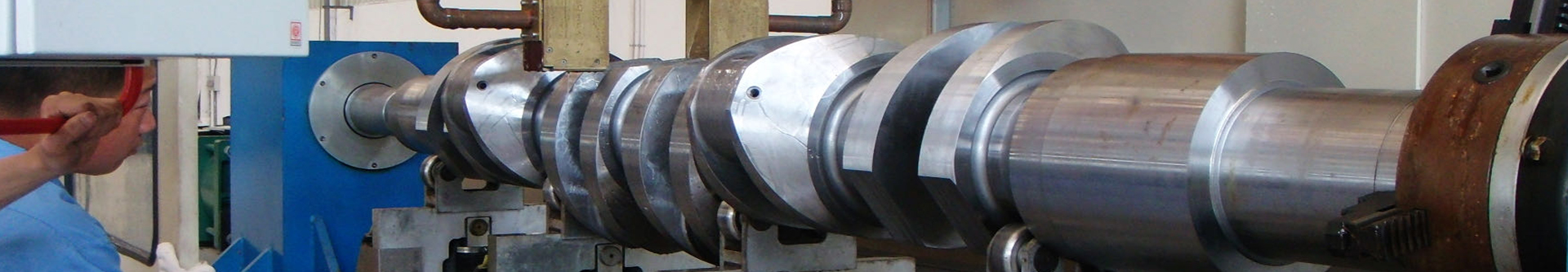


 GS-ZP-1200
GS-ZP-1200